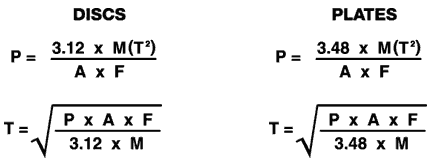|
 quartz glass
quartz rod, fused silica fused quartz,quartz wool, quartz tubing,
quartz tubing, quartz rod, Silica tubing, fused Quartz, silica rod quartz glass
quartz rod, fused silica fused quartz,quartz wool, quartz tubing,
quartz tubing, quartz rod, Silica tubing, fused Quartz, silica rod
|
fused quartz,
fused silica, quartz tubing, quartz rod, glassblowing, quartz wool,
quartz glass
quartz rods, heat resistant glass,
silica glass, fused glassware, spectrophotmeter cells
quartzglas, cuvettes, quartz cells,
flourimeter cells laboratory glassware, polished quartz,
| products |contact
us | find
us| partners
|home | |
fused quartz,
fused silica, quartz tubing, quartz rod, glassblowing, quartz wool,
quartz glass
quartz rods, heat resistant glass,
silica glass, fused glassware, spectrophotmeter cells
quartzglas, cuvettes, quartz cells,
flourimeter cells laboratory glassware, polished quartz, |
|
products
contact
us
find
us
partners
home
|
|
PROPERTIES OF
FUSED QUARTZ
Silica
is found almost everywhere in nature, it represents almost 1/3
the mass of the earth’s crust. Vitreous Silica is the generic
term used to describe all types of silica glass, with
manufacturers referring to the material as either Fused Quartz
or Fused Silica.
Manufactured
by fusing naturally occuring crystalline silica, either sand or
rock crystal, a wide range of products are available. After this
process, their appearance wil be wither opaque, translucent or
transparent. If the silicon dioxide is synthetically derived,
the material produced is commonly called Synthetic Fused Silica.
Vitreous
Silica, in all its forms, offers a variety of properties such
as:
- Permeability
- Extreme
Hardness
- Very
Low Coefficient of Thermal Expansion
- Resistance
to High Temperature
- High
Chemical Purity
- High
Corrosion Resistance
- Extensive
Optical Transmission from Ultra-Violet to Infra-Red
- Excellent
Electrical Insulation Qualities
- Remarkable
Stability Under Atomic Bombardment
|
|
|
|
 |
 |
 |
|
PROPERTY
|
ENGLISH
& METRIC
SYSTEM VALUE
|
INTERNATIONAL
SYSTEM
OF UNITS (SI) VALUE
|
| Density |
2.2
gm/cm3 |
2.2
x 103 kg/m3 |
Hardness
|
5.5–6.5
Mohs’ Scale
570KHN100 |
|
| Design
Tensile Strength |
7,000
psi |
4.8
x 107 Pa (N/m2) |
| Design
Compressive Strength |
Greater
than 160,000 psi |
Greater
than 1.1 x 109 Pa |
| Bulk
Modulus |
5.3
x 106 psi |
3.7
x 1010 Pa |
| Rigidity
Modulus |
4.5
x 106 psi |
7.2
x 1010 Pa |
| Young’s
Modulus |
10.5
x 106 psi |
7.2
x 1010 Pa |
| Poisson’s
Ratio |
.17 |
.17 |
Coefficient
of Thermal Expansion
|
5.5
x 10-7 cm/cm • °C
(20°C – 320°C) |
5.5
x 10-7 m/m • °K
(293°K – 593°K) |
| Thermal
Conductivity (20° C) |
3.3
x 10-3 gm cal • cm/cm2 • °C |
1.4
W/m • °K |
| Specific
Heat (20°) |
.16gm
cal/gm |
670
J/kg • °K |
| Softening
Point |
1683°C |
1956° |
| Annealing
Point |
1215°C |
1488° |
| Strain
Point |
1120°C |
1393° |
Electrical
Resistivity
|
7(107)
ohm • cm
(350°C) |
7(107)ohm-m
|
| Dielectric
Properties |
(20°C
and 1 MHz) |
(293°K
and 1 MHz) |
| Constant |
3.75 |
3.75 |
| Strength |
5
x 107 volts/mil |
5
x 107 V/m |
| Loss
Factor |
Less
than 4 x 10-4 |
Less
than 4 x 10-4 |
| Dissipation
Factor |
Less
than 1 x 10-4 |
Less
than 1 x 10-4 |
| Index
of Refraction |
1.4585 |
1.4585 |
Constrigence
(Nu value)
Fused Quartz |
67.56 |
67.56 |
|
Velocity of Sound-Shear Wave |
3.75
x 105 cm/sec |
3.75
x 103 m/s |
|
Velocity of Sound-Compression Wave |
5.90
x 105 cm/sec |
5.90
x 103 m/s |
|
Sonic Attenuation |
Less
than 11 db/m • MHz |
Less
than 11 db/m • MHz |
| Permeability
Constants |
(cm
• mm/cm • sec • cm of Hg – 700°C/973°K) |
| Helium |
210
x 10-10 |
| Hydrogen |
21
x 10-10 |
| Deutrium |
17
x 10-10 |
| Neon |
905
x 10-10 |
|
|
|
|
|
TRACE
IMPURITIES |
|
TYPE
(PPM)
|
AI
|
AS
|
B
|
Ca
|
Cd
|
Cr
|
Cu
|
Fe
|
K
|
Li
|
Mg
|
Mn
|
Na
|
Ni
|
P
|
Sb
|
Ti
|
Zr
|
*OH-
|
|
GE
124®
|
14
|
<.002
|
<0.2
|
0.4
|
<0.01
|
<0.05
|
<0.05
|
0.2
|
0.6
|
0.6
|
0.1
|
<0.05
|
0.7
|
<0.1
|
<0.2
|
<0.003
|
1.1
|
0.8
|
<5
|
|
GE
214®
|
14
|
<.002
|
<0.2
|
0.4
|
<0.01
|
0.05
|
<0.05
|
0.2
|
0.6
|
0.6
|
0.1
|
<0.05
|
0.7
|
<0.1
|
<0.2
|
<0.003
|
1.1
|
0.8
|
<5
|
|
NSG
OZ®
|
40
|
–
|
–
|
2.5
|
–
|
–
|
.50
|
0.9
|
1.7
|
.06
|
0.3
|
.03
|
2.5
|
–
|
–
|
–
|
0.8
|
–
|
150–200
|
|
TYPE
(PPB)
|
Ag
|
Al
|
As
|
Au
|
B
|
Ba
|
Be
|
Bi
|
Ca
|
Cd
|
Co
|
Cr
|
Cu
|
Fe
|
Ga
|
|
|
Corning
7980 ®
|
<150
|
-40
|
<5
|
n.d.
|
<100
|
<14
|
<5
|
<10
|
<20
|
n.d.
|
<10
|
<1
|
<13
|
<15
|
n.d.
|
|
|
K
|
Li
|
Mg
|
Mn
|
Mo
|
Na
|
Ni
|
P
|
Sb
|
Sr
|
Ti
|
U
|
V
|
Zn
|
Zr
|
|
|
<21
|
<1
|
<25
|
<10
|
<5
|
<150
|
<7
|
<100
|
<5
|
<3
|
<40
|
<1
|
<10
|
<30
|
<30
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INTERNAL
PRESSURE CALCULATIONS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
RUPTURE
FORMULA FOR TUBING |
|
|
|
|
|
|
|
|
|
|
|
|
Because
fused quartz is used in applications involving internal
pressures, it is helpful to know the maximum pressure that can
be applied to a selected fused quartz tube. The formula at right
can approximate this information at room temperature. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S
= pr/t |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Where: |
|
S
= Hoop Stress in Pa |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
p
= Working Pressure (Pa) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
r0
= Inside Radius (mm) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
t
= Wall Thickness (mm) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
formula can not be used when internal pressure exceeds 100 psi.
|
|
|
|
|
|
|
|
|
RUPTURE
PRESSURE CALCULATIONS FOR DISCS AND PLATES |
|
|
|
|
|
|
|
Determining
pressure differential is required for many applications of
stressed fused quartz discs, plates and sight glasses. The
formulas below can be used for room temperture applications of
parts having either clamped or unclamped edges. |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A =
Unsupported Area in sq/inches
T =
Thickness (inches)
F =
Safety Factor (7)
M =
Modulus of Rupture (7,000 psi)
P =
Pressure (psi)
|
|
|
|
|
|
|
|
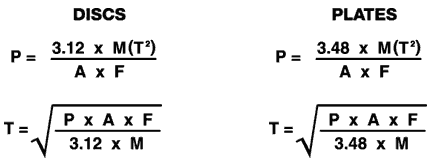 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
THE ABOVE
PRESSURE CALCULATIONS ARE RECOMMENDATIONS ONLY.
ACTUAL PRESSURE POINTS MAY VARY DEPENDING ON USER APPLICATIONS
|
|
|
|
| |
|
|
|
FUSED
QUARTZ PROPER USAGE GUIDELINES
|
|
CLEANING
The
cleaning of fused quartz is critical before it is used
in any application. The fused quartz should be cleaned
by placing it in a 7% maximum solution of Ammonium
Bifluoride for no more than ten (10) minutes, or a 10%
volume maximum solution of Hydrofloric Acid for no more
than five (5) minutes. After cleaning, using the above
method, the fused quartz should be rinsed in deionized
or distilled water and then dried.
RUNNING
IN PROCEDURE
In
order to increase resistance to devitrification and sag
of your quartzware, an even layer of cristobalite must
be formed on the O.D. of quartz tubes. Expose a new tube
to a temperature of up to 1200° C and rotate it 90°
every two (2) hours for the first 12 to 24 hours.
STORAGE
Space
permitting, fused quartz should be stored in its
original shipping container. If that is not practical,
at least the wrapping should be retained. In the case of
tubing, the end coverings should be kept in place until
the product is used. This protects the ends from
chipping and keeps out dirt and moisture which could
compromise the purity and performance of the tubing.
BECAUSE
THE PRODUCTS ARE ANNEALED
Both
quartz and silica glass are annealed at approximately
1150° C. However, they reach a strain point at about
1120° C. These glass products, if rapidly cooled after
use at temperatures above this strain point, will
develop strain again. Special care should be taken when
using large sized products.
WHEN
JOINING FUSED QUARTZ AND OTHER MATERIALS
Quartz
and silica glass only slightly expand with increases in
temperature, in contrast with other materials. Care must
be taken when these glass products are connected to
other materials and the temperature rises, in order to
avoid the development of cracks.
CARE
MUST BE TAKEN DURING FURNACE INSERTION
Quartz
and silica glass feature low thermal conductivity. If
the glass product comes too close to a heating element,
or is put in direct contact with a flame, it may become
locally heated and develop cracks. Long glass tubes may
also deform at temperatures of 1100° C or higher. Care
should be taken to support both glass types, expecially
large-sized products.
DEVITRIFICATION
Devitrification
of quartz and silica glass means transition from a
metastable (vitrified) state to a stable crystallized
state of cristobalite. Devitrification occurs when the
product is used at high temperatures over a long period
of time, or it is heated while impurities adhere to its
surface. Even very small impurities on the surface can
have a major influence. Under such conditions,
devitrification may even occur at temperatures of 1000°
C or less. This hardly ever occurs at temperatures of
1150° C or less, if the glass surface is perfectly
clean. Devitrification usually starts when the
temperature rises to 1200° C or higher, then further
develops as the temperature increases.
|
|
-
-
-
- To request
further information or a quotation please contact our sales team
-
- Telephone
- 00 44 (0)1473
626585
- FAX
- 00
44 (0)1473 610421
- POSTAL
ADDRESS -
30Anson Road, Martlesham Heath, Ipswich, Suffolk, England,
- IP5 3RG.
- Electronic
mail - sales@h-baumbach.co.uk
|
|